
The bond angle for trigonal pyramidal geometries is less than due to the additional repulsion from the lone pair. The bond angle for tetrahedral molecules is approximately trigonal pyramidal geometry exists when there are 3 bonds and 1 lone pair. For example, `CH_4` adopts a tetrahedral geometry (left). This is one of the most important and common geometries, as many molecules will adopt this. The tetrahedral geometry exists when there are 4 bonds and 0 lone pairs. This group consist of tetrahedral, trigonal pyramidal, and bent geometries. Therefore, the lone pair in a bent molecule takes up more room than the 3rd bond in a trigonal planar molecule does, thereby reducing the angle to slightly less than Electron Groups

In bent molecules, the bond angle is slightly less than This is because lone pairs take up more room than single bonds do. In a trigonal planar molecule, there are 3 bonds and 0 lone pairs, with bond angles of Bent molecules have 2 bonds and 1 lone pair. We've gone over examples of these two: `BF_3` is a trigonal planar molecule whereas water is a bent molecule. This group consists of trigonal planar molecules and bent molecules.
All linear molecules share the property that they are completely straight i.e the bond angle from one bond to another is Electron Groups Notice that there will be several different linear geometries. Linear geometries occur when there are only 2 bonds and 0 lone pairs. This group consists only of the linear geometry. Now let's go over the geometries in terms of the number of electron pairs: From all outward appearances, they will look identical however. For example, both molecules with 2 bonds and 0 lone pairs and 2 bonds and 3 lone pairs will be linear. They will have different numbers of bonds and lone pairs, but the end result will be the same. One brief thing to take note of is that there are multiple linear and bent geometries. You'll see that the chart follows a very straightforward pattern of logic. The molecular geometry chart may seem like a lot of information at first, but over time it will become intuitive. The molecular geometry for a water molecule is bent, which is why the water molecule isn't a straight H-O-H molecule. If we look up at the table, we can find on the left side a column for molecules with 4 electrons groups, and within that group a molecule with 2 lone pairs. The central `O` atom has 4 groups of electrons around it: 2 bonds and 2 unpaired electrons, also called lone pairs. Let's say you had the formula of water, `H_2O`, and wanted to know its molecular geometry. In other words, by drawing out the Lewis structure of a molecule, one can determine the molecule's 3d orientation. We're going to discuss each one individually, but note that you can determine the molecular geometry of a molecule solely by the number of bonds and lone pairs around the central atom. The following chart provides the different molecular geometries and the conditions in which they arise. This will become important when discussing bond angles. If you substitute a single bond with a lone pair, the lone pair will repel the other electron groups away from it more than the single bond would. One key point is that lone pairs take up more room than single bonds do.

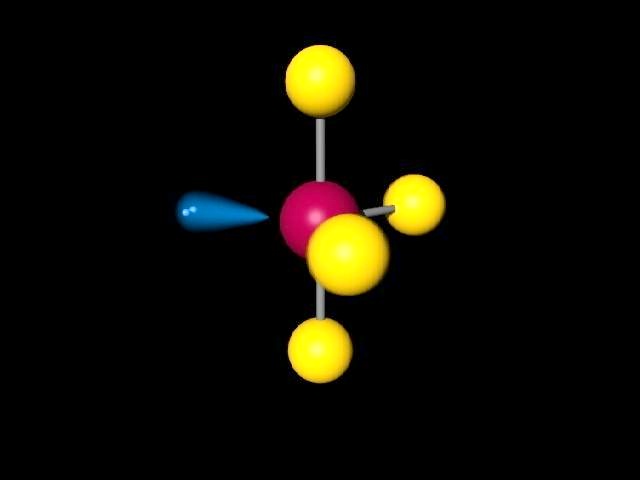
The lone pair is usually not shown in 3d models and thus you have to visualize it yourself. This is why `NH_3` has a tri-pod shape: the lone pair on top of `N` pushes the other electrons (in the bond) away from it. The basic idea behind VSEPR is that electron pairs will repel each other. The only difference is the number of electrons on the central atom: `N` has a lone pair whereas `B` does not. Take a look at the Lewis structures of `NH_3` and `BF_3`. Molecular geometry is determined by the arrangement of valence electrons. The reason behind this is that the two structures have a different number and arrangement of valence electrons. This is the case of `BF_3` and `NH_3`: both have the general formula `AB_3`, but `NH_3` on the left is a tri-pod shape whereas `BF_3` on the right is a 3-legged starfish shape. In other words, two molecules with the general formulas `AB_3` may look completely different in real life: one may be a pyramid whereas the other may be completely flat. Valence Shell Electron Pair Repulsion (VSEPR) is a theory that states that the 3d orientation, also known as the molecular geometry, of a molecule is not dependent on its chemical formula but on the repulsion of valence electrons.


 0 kommentar(er)
0 kommentar(er)
